Ribonucleotide reductase M2B in the myofibers modulates stem cell fate in skeletal muscle (NPJ Regen Med ,2022 Jul 29)
Dr.Yi-Fan Chen
Instead of prolonging lifespan, scientists have long been in pursuit of maintaining human healthspan. To keep the ideal quality of life is especially crucial for the elderly since the declined muscle mass and strength caused physical inconvenience. Therefore, maintaining the health of skeletal muscle is of vital importance.
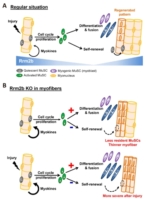
Professor Yi-Fan Chen and Professor Yun Yen from Taipei Medical University have recently published their work in npj regenerative medicine. The research article elaborated how Ribonucleotide reductase M2B (Rrm2b) modulates the fate of stem cells in skeletal muscle in response to injury. The homeostasis of skeletal muscle relies on the interplay between the muscle stem cells (MuSCs) and their microenvironment (niche). By genetically modified mouse models, Chen unveiled that specific knockout of Rrm2b in the myofibers (a part of niche), but not in MuSCs, led to the weakness of muscles, including loss of muscle mass and strength. These Rrm2b myofiber-specific knockout mice displayed compromised regenerative capacity of muscle with thinner fiber sizes and weaker functioning. Moreover, the lack of Rrm2b in the myofibers resulted in mitochondrial defects, showing a part of the typical characteristics of mitochondrial myopathy.
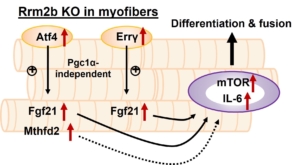
Furthermore, Chen’s team collaborated with Dr. I-Hsuan Lin for RNA-sequencing to identify several myokines released from Rrm2b-deleted myofibers. These myokines, including FGF-21, GDF-15, and Mthfd2, triggered MuSCs differentiation rather than reentry of quiescence to repopulate the stem cell pool. The decreased MuSC pool due to the imbalance between differentiation and self-renewal of MuSCs thus contributed to muscle weakness and impaired regenerative capacity.
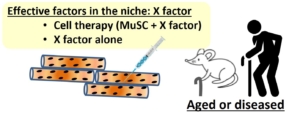
In conclusion, Chen’s study identified a novel role of Rrm2b in muscle homeostasis. Rrm2b in the myofibers plays a critical role in modulating the stem cell fate of MuSCs by an alternation of the microenvironment (niche), and it provides an opportunity for strategy development to treat muscle disorders. Animals with defective Rrm2b expression can probably serve as a disease model for investigating mitochondrial myopathy in mammals. With these striking research results, promoting muscle health in clinical use can be expected in the coming years.